Senseonics’ glucose monitoring system gets FDA approval
FDA has given approval for the Germantown, MD-based company Senseonics’ device Eversense CGM system to be inserted by trained medical practitioners in diabetes patients of the age 18 and above.
The Germantown, MD-based company Senseonics Holdings finally managed to receive a go-ahead from FDA of United States to introduce its Eversense Continuous Glucose Monitoring (CGM) system in US markets. This approval has opened many doors, and it allows the competent healthcare providers to train and get a certification to use Eversense with the patients, which will last up to 90 days or three months. Earlier, only the trained physicians were allowed to perform the sensor insertion and removal procedure. The device has already been on the European markets for a little over two years.
Senseonics received the FDA approval for the Eversense CGM system to use on the diabetes patients who are 18 or above.
This is the first FDA-approved CGM system which comprises of a completely implantable sensor to detect the glucose readings and can be worn for up to 90 days.
According to what Tim Goodnow, president, and CEO of Senseonics said in a press release that after receiving the FDA approval, the company is looking to include nurse practitioners and physician assistants in their list of diabetes care professionals who can be trained and certified to use the Eversense CGM System on the patients.
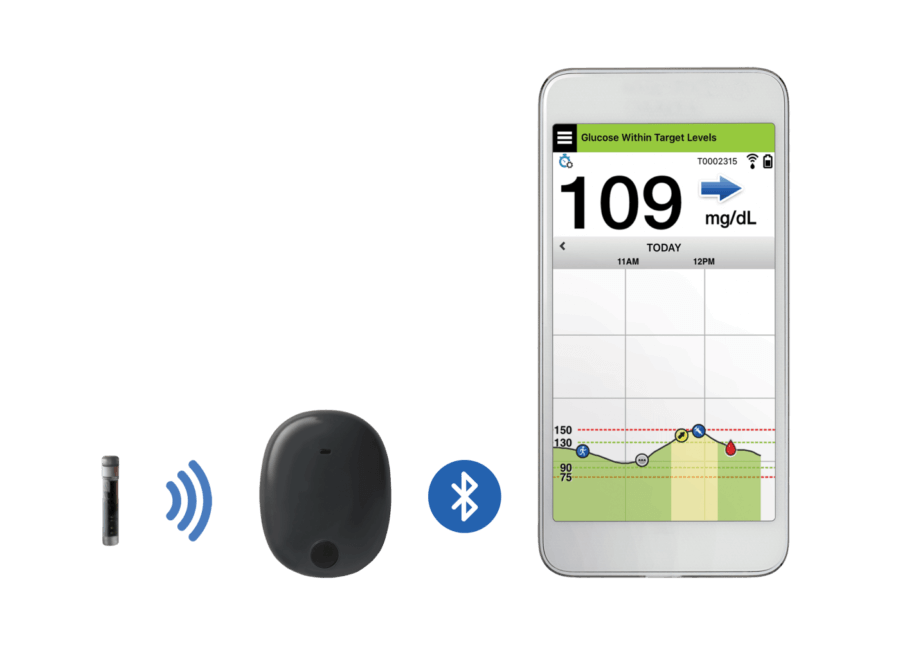
The Senseonics’ Eversense CGM System comprises of a fluorescence-based sensor, an intelligent and smart transmitter worn over the sensor to enable the data communication, and a mobile app which will show the glucose levels, trends, and alerts.
FDA commissioner Scott Gottlieb MD said in a statement that the FDA is dedicated to moving forward with the innovative products that will help digital technology in improving the patient care services. He further added that these innovative technologies will help the patients get an improved control over their overall health condition and an enhanced ability to successfully manage chronic diseases like diabetes.
The Eversense CGM system uses a small sensor, which is inserted just underneath the skin by a trained and skilled health care provider during a procedure. It is done with the aim of measuring the glucose levels in adult diabetics on a regular basis for up to 90 days.
This implanted sensor uses an innovative light-based technology to measure blood glucose levels, and then send the gathered data to a mobile app in order to alert the users in case their glucose levels are too low or too high.
The sensor is covered with a fluorescent chemical. When this chemical gets exposed to the blood sugar, it generates a small amount of light which is then measured by the sensor. In every 5 minutes, the measurements are delivered to a connected mobile device running a device-specific mobile app. It takes only 5 minutes for the trained physician to insert and remove the sensor from the body using a local anaesthetic, leaving only a small cut behind.
As stated by the FDA, the agency assessed experimental study data collected from 125 individuals with diabetes who were 18 and above it. FDA studied the effectiveness of the device by comparing the readings that were acquired using the Eversense CGM system to the readings that were obtained using a laboratory-based glucose analyzer.
The safety of the sensor and the procedure to implant it were also evaluated. During those studies, FDA found that the percentage of the individuals undergoing serious adverse effects with the implanted sensor was below 1 percent. FDA will also be monitoring the safety of the CGM system in a post-approval study.
Image credit: www.eversensediabetes.com